Auto-propose can help you quickly slender down your search engine results by suggesting possible matches as you kind.
A CQA is a Bodily, chemical, biological or microbiological residence or characteristic that should be inside an proper Restrict, range, or distribution to guarantee the desired product good quality.
When you got a PhD diploma, but experienced no a lot more expertise in the subject with the degree Apart from what you've now, what degree would you wish to be offered to you?
Managed / planned deviation: Any deviation from documented procedure opted deliberately for non permanent time period to deal with unavoidable predicament or improving the general performance in the operations, without influencing the quality & yield of drug material and protection in the functions shall be termed as managed / prepared deviation.
The quantity of batches to be taken under validation depends on the chance involved with the production Essential course of action parameters & crucial Good quality Attribute so is dependent upon that company have to select the amount of batches to be validated.
among two or even more rooms, e.g. of differing classes of cleanliness, for the objective of managing the airflow among Those people rooms when they have to be entered.
We made a nanoemulsion process which significantly elevated the area spot, improving the dissolution rate and enhancing absorption during the gastrointestinal tract.
Dried granules are again screened via a sieve which helps it to break down the granule then it should be lubricated or mixed in Blender. These exact same size Blend are then compressed or click here can be filled in capsule.
I scrutinized Just about every phase, from raw content collection to reaction ailments. Via systematic Evaluation, it grew to become apparent that impurities were introduced for the duration of an previously section which interfered with the ultimate synthesis.
Re-test date: The day when a cloth needs to be re-examined to make certain it is still appropriate for use. The time period all through which the drug substance is predicted to stay inside its specs and for that reason, can be utilized during the manufacturing from the drug product or service, provided that drug substance is saved beneath the described problems.
Dedicated equipment: It is actually used entirely to the creation of one item or item line. Issues about cross-contamination with other goods are markedly lessened.
Accelerated balance tests is done at elevated temperatures and humidity stages to predict the drug’s shelf life inside of a limited period.
What was the final situation the place some Odd things went down and everyone acted like it had been ordinary, so you weren’t sure question forums in case you had been ridiculous or Everybody close to you was outrageous?
Do you think silence is enjoyable or unnerving? How come you think that other people could possibly experience the other way?
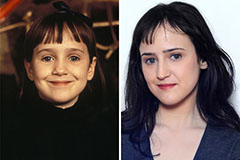 Mara Wilson Then & Now!
Mara Wilson Then & Now! Alexa Vega Then & Now!
Alexa Vega Then & Now! Michael C. Maronna Then & Now!
Michael C. Maronna Then & Now! Macaulay Culkin Then & Now!
Macaulay Culkin Then & Now! Jeri Ryan Then & Now!
Jeri Ryan Then & Now!